Back
Isotopes
Atoms of the same element always have the same number of protons (or have the same atomic number). But atoms of the same element can have different numbers of neutrons: these atoms are known as isotopes.
**Examples **
- Protium is a hydrogen atom with 1 proton and 0 neutrons. 99.98% of hydrogen atoms are protium. It is used in hydrogen fuel cells and the production of plastics.
- Deuterium is a hydrogen atom with 1 proton and 1 neutron. Around 0.02% of hydrogen atoms are deuterium. It is used in nuclear fusion.
- Tritium is a hydrogen atom with 1 proton and 2 neutrons. It is very rare. It is used in thermonuclear fusion weapons.
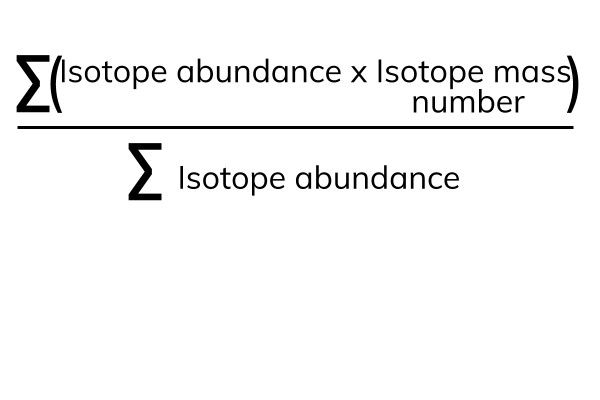
Relative Atomic Mass (Ar)
The relative atomic mass (Ar) is the average mass of all of the isotopes of an element. It takes into account how often each isotope is found (the isotope abundance).
The Σ means 'sum of'. The numerator is 'the sum of the isotope abundance times the isotope mass'. The denominator is 'the sum of all the isotope abundances'.
Want to learn more about Isotopes?
Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses.





